TP9 – Stable immobilization of electrocatalysts and design of modified electrodes towards selective glycerol electrooxidation
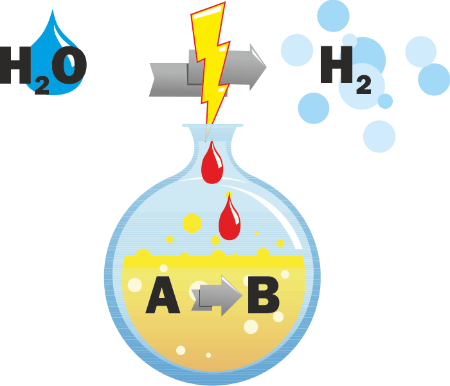
Hydrogen synthesis through water electrolysis is hindered mainly by the high overpotentials associated with the anodic oxygen evolution reaction (OER). Replacement of the OER by other oxidation reactions and development of electrocatalyst materials which catalyze these reactions is aimed in the present research unit. Stable and active catalysts, which allow the selectively oxidation of complex molecules like glycerol to industrial valuable compounds, will be developed directly on the electrodes by embedding the previously synthesized inorganic catalysts (provided by TP8 and TP1) in a specifically selected polymer matrix, which will be later converted into a conductive carbon support by means of a thermal treatment. The stable immobilization of the catalyst materials in carbon composites is aimed in a first step, with a potential to expand the concept to special arrangements of different active centers, which can allow cascade reactions to occur, increasing thus selectivity. Evaluation of activity, stability and selectivity of different non-noble metal based electrocatalysts in different alcohol electrooxidation reactions is the goal, especially in collaboration with TP8 and TP1. Mechanistic investigations will be performed in collaboration with TP1 and TP7. By specific arrangement of catalysts which exhibit different selectivity in layers directly on the electrode, or by tuning the reaction conditions, synergistic interaction and follow-up conversion of primarily formed products will allow cascade reactions to occur as a key to control and modulate selectivity of the overall reaction. In the first funding period, the cascade reaction concept will be explored for the selectively electroreduction of glycerol to lactic acid by combing an active electrocatalyst material which allows the selective formation of glyceraldehyde by glycerol electrooxidation and prevents its further electrooxidation, with suitable reaction conditions which facilitate the follow-up chemical conversion of glyceraldehyde to lactic acid.
Andronescu, Corina, Jun.-Prof. Dr. -Ing., born 14.02.1987, Romanian; AN1570
Faculty of Chemistry, Technical Chemistry III and Center for Nanointegration (CENIDE), University of Duisburg-Essen,
Carl-Benz-Straße 199, D-47057 Duisburg, Germany
Tel.: +49 1578 1023612; Email: corina.andronescu@uni-due.de

 UNODE- DFG Forschergruppe 2982
UNODE- DFG Forschergruppe 2982